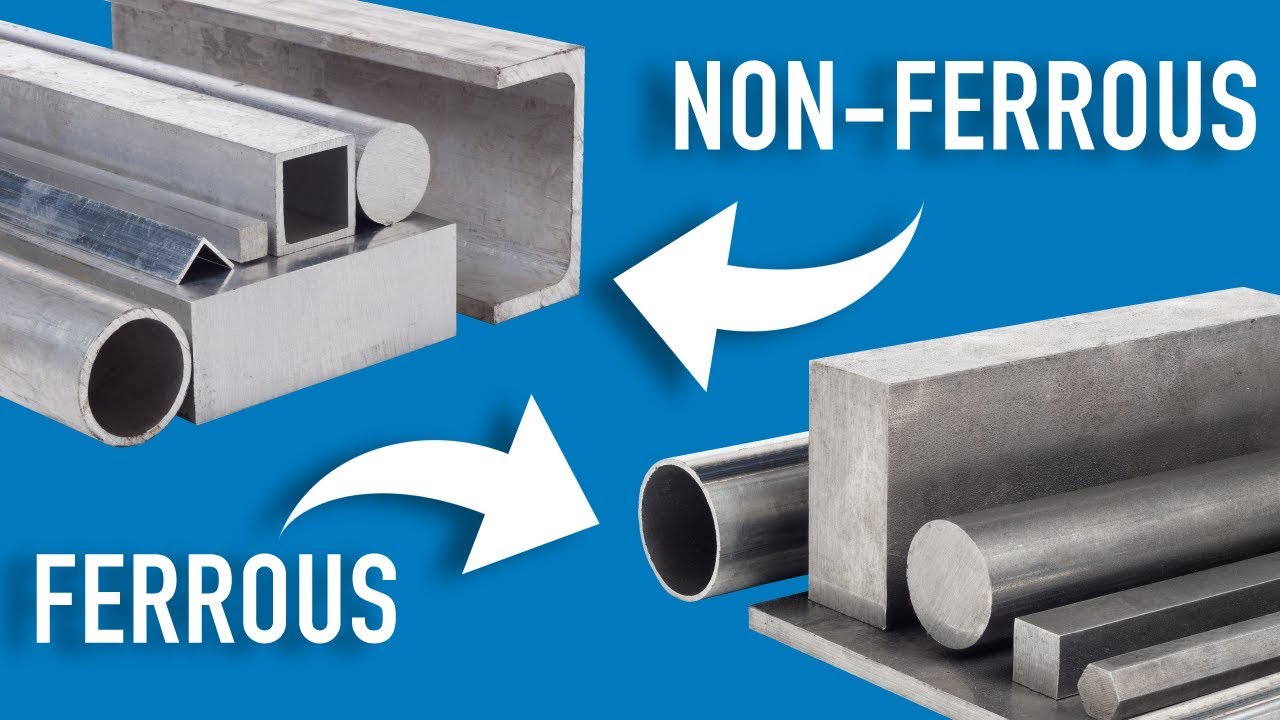
Recycling metals is an important component that would enable conservation of the natural environment as well as help to reduce wastage of valuable resources. Metal products and components are considered one of the most tradable or recyclable materials across the world since recycling metals is one way of saving vast amounts of energy as well as reducing the amount of waste that is chucked away from the environment. However, the use of recycling involves a major factor that is sorting ferrous and non-ferrous metals. Such a difference guarantees cost-effectiveness, the quality of the used materials being high, and economic and environmental effects.
Table of Contents
ToggleWhat Are Ferrous and Non-Ferrous Metals?
Ferrous metals contain iron. Steel, cast iron, and wrought iron are a few of these. While these metals are magnetic, it must be noted that when they are not alloyed with material such as chromium or nickel to prevent them from rusting when wet. Non-ferrous means that they do not contain iron. These are materials such as aluminum, copper, brass, zinc, and lead. Non-ferrous metals are characterized by being light, having high resistance to corrosion, and having no appealing properties apart from these characteristics, making them very versatile across the industrial and commercial markets.
Comparing Ferrous and Non-Ferrous Metals
Because ferrous and non-ferrous metals have different characteristics, each kind is appropriate for a certain use. Due to their strength and magnetic properties, ferrous metals are frequently utilized in infrastructure and building projects. Non-ferrous metals, which are lightweight and resistant to rust, are often preferred for applications like electrical wiring, aerospace components, and packaging. Additionally, nonferrous metals tend to have a higher market value due to their desirable properties and limited availability.
Separating Metals During Recycling is Vital
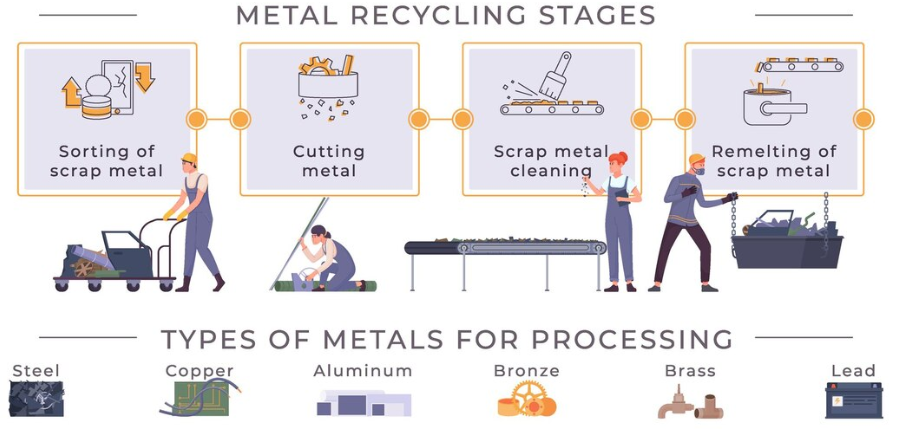
The separation of ferrous and non-ferrous metals is essential for several reasons.
Firstly, separating metals preserves their unique properties. Ferrous metals, when mixed with non-ferrous counterparts, can contaminate the latter, reducing their quality and suitability for high-precision applications. For instance, aluminum, widely used in industries such as aerospace and automotive, requires a high degree of purity to meet performance standards.
Secondly, proper separation enhances recycling efficiency. Each type of metal requires distinct processing techniques. Ferrous metals, which melt at high temperatures, are processed differently from non-ferrous metals like aluminum or copper, which have lower melting points. Mixing these metals can complicate the recycling process, leading to inefficiencies and increased waste.
Economic value is another key factor. Non-ferrous metals typically have a higher market price than ferrous metals. Copper, for example, is far more valuable than steel due to its electrical conductivity and corrosion resistance. When non-ferrous metals are mixed with ferrous materials, the overall value of the scrap decreases, leading to financial losses for recycling facilities and reducing the incentive to recycle.
Environmental benefits also underscore the importance of separation. Recycling metals reduces the need for mining raw materials, conserving natural resources, and lowering energy consumption.Recycling aluminum, for instance, can save up to 95% of the energy needed to make it from ore. However, by raising the energy needed to treat contaminated materials, incorrect separation might negate these advantages.
How Are Metals Separated?
Metal separation starts with magnetic sorting. It is a simple technique for distinguishing ferrous metals. Since ferrous metals are magnetic, they can be easily extracted from mixed scrap using magnets. Non-ferrous metals are separated through other methods, including eddy current separation. This method induces a magnetic field that repels non-ferrous metals and thus separates them.
Manual sorting is also carried out, though on a relatively smaller scale. Employees visually inspect metals and determine their respective identities by the color, weight, and texture of metals. Advanced technologies like X-ray fluorescence and spectrometry in contemporary recycling plants identify the chemical makeup of metals and classify them precisely.
Examples of Recycling Success Stories
Copper recycling is a clear-cut example of the gains made through proper metal separation. Copper is a very conductive metal and is reused enormously in electrical wiring and in electronic components. Its utility can thus be maximized by keeping its purity during recycling.
Another significant importance of separation is reflected in aluminum recycling. Beverage cans are one of the most common sources of aluminum waste. It can be recycled infinitely without loss of quality. Separation ensures that aluminum remains clean and ready for reuse in packaging, construction, and transportation.
Conclusion
Separation of ferrous and non-ferrous metals is a fundamental principle of effective recycling. It ensures that each type of metal maintains its valuable properties, maximizes the processes of recycling, and increases the economic and environmental advantages of reusing metals.
Separation of metals before recycling is a step that is easy but effective for both individuals and institutions. Separation can be much facilitated by distinguishing ferrous from non-ferrous metals with a magnet and keeping them sorted.
All of these practices help ensure that a better, more sustainable future is ensured wherein resources are conserved and waste is kept at a minimum while recycling continues to benefit both the economy and the environment.